Abstract
INTRODUCTION Risk-adapted therapies based on cytogenetic and molecular markers are being increasingly complemented by targeted therapies, such as FLT3-TKD, FLT3-ITD, IDH1/2 inhibitors. Together, risk-adapted and targeted strategies may help to improve the clinical outcomes of AML patients. However, although extensive genetic and molecular panel screening is nowadays recommended at diagnosis, these prognostic markers or actionable mutations may not always be available in routine practice, and therefore a proportion of patients will not be screened. As of today, there is little information on evolving diagnostic practices based on epidemiologic registry data.
AIMS: The REALMOL study describes main genetic studies performed at diagnosis in adult AML patients included in the PETHEMA AML registry through last 20 years.
METHODS We conducted a retrospective study to provide insights into genetic and molecular diagnostic methods performed at diagnosis in routine clinical practice in adult AML (non-APL) patients included in the Spanish PETHEMA Registry (NCT02607059), between 2000 and October 2021. All AML patients with available information on both FLT3-ITD and NPM1 test (performed/not performed) were eligible. The primary endpoint of the study was the percentage of screened patients for genetic (karyotype and Fluorescence in situ hybridization) and molecular analysis (conventional PCR for NPM1 and FLT3-ITD, and next-generation sequencing [NGS]) over time, according to patient characteristics, and the type of AML.
RESULTS A total of 7,285 adult AML patients were eligible, 4023 patients were male (55.3%), and median age was 66 years (18-99). Two thirds of patients (n=4816) had de novo AML, and ECOG performance status was <2 in 72%. Therapeutic approach was intensive chemotherapy (IC) in 63%, attenuated therapy (non-IC) in 21%, and best supportive care (BSC) in 16% of patients.
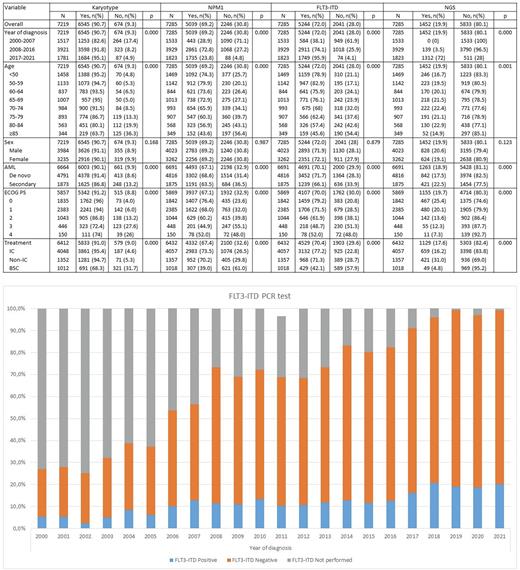
Throughout the entire period, karyotype was performed in 91% of patients, Fluorescence in situ hybridization (FISH) in 70.3%, NPM1 in 69.2%, FLT3-ITD in 72.0%, and NGS in 19.9% of patients (Table 1).
The percentage of screened patients for all genetic and molecular analyses increased over the different periods (2000-2007, 2008-2016 and 2017-2021) (Table 1). Regarding FLT3-ITD determination, it was 82.6% in 2000-2007 (most were performed retrospectively), 91.8% in 2008-2016, and 95.1% in 2017-2021 (Figure 1). Regarding NGS testing, 0.1% in 2000-2007 (most were performed retrospectively), 3.5% in 2008-2016, and 72% in 2017-2021.
Age, type of AML (de novo vs. secondary), ECOG and therapeutic approach (BSC vs. IC or non-IC) also significantly influenced the percentage of genetic and molecular studies performed at diagnosis (Table 1). In a multivariate model including all these variables, therapeutic approach (BSC vs. IC/non-IC) was the most relevant factor for not requesting a karyotype study (O.R.: 6.057, 95%C.I. 4.702-7.802, p<0.0001), followed by ECOG ≥2 vs 0-1 (O.R.: 1.733, 95%C.I. 1.388-2.165, p<0.0001), age at diagnosis ≥70 years vs <70 years (O.R.: 1.520, 95%C.I. 1.184-1.950, p=0.001) and secondary vs. de novo AML (O.R.: 1.279, 95%C.I. 1.031-1.588, p=0.025).
Regarding FLT3-ITD, the year of diagnosis (<2017 vs ≥2017) was the most influential factor (95% CI 8.408-14.494, p<0.0001), followed by treatment strategy (BSC vs. IC/non-IC, OR: 2.393, 95% CI 1.994-2.873, p<0.0001), age at diagnosis ≥70 years (OR: 1.766, 95% CI 1.537-2.030, p<0.0001), ECOG ≥2 (OR: 1.285, 95% CI 1.114-1.481, p=0.001) and secondary AML (OR: 1.191, 95% CI 1.037-1.369, p=0.014).
The year of diagnosis was also the most important factor in the performance of the NGS study (<2017 vs ≥2017) (or 75.713, 95%CI 60.840-94.221, p<0.0001), followed by treatment with BSC (OR 4.287, 95% CI 2.835-6.483, p=0.000), age <70 years (OR 2.374, 95% CI 1.888-2.985, p=0.000) and ECOG ≥2 (OR: 1.365, 95% CI 1.049-1.776, p=0.020).
CONCLUSIONS Our study provides relevant information about the diagnostic use of the main genetic and molecular markers in AML in the real-life. The use of all genetic and molecular analysis increased from 2000 to 2021, reflecting complexity of AML diagnostic needs. Age, type of AML, ECOG and therapeutic approach also influenced the type of diagnostic studies performed.
Disclosures
Perez-Simon:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ABBVIE: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; GILEAD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; JAZZ: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; ALEXION: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, and Expenses; PFIZER: Research Funding. Bergua Burgués:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Ramos:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Astellas: Consultancy, Honoraria; Sandoz: Honoraria; Jazz: Honoraria. Bilbao:Astellas: Other: Speakers. Montesinos:OTSUKA: Consultancy; KURA ONCOLOGY: Consultancy; ABBVIE: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; NOVARTIS: Consultancy, Research Funding, Speakers Bureau; RYVU: Consultancy; TAKEDA: Consultancy, Research Funding; INCYTE: Consultancy; PFIZER: Consultancy, Research Funding, Speakers Bureau; ASTELLAS: Consultancy, Speakers Bureau; BEIGENE: Consultancy; JAZZPHARMA: Consultancy, Research Funding, Speakers Bureau; GILEAD: Consultancy, Speakers Bureau; MENARINI/STEMLINE: Consultancy, Research Funding; NERVIANO: Consultancy. Alonso-Dominguez:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal